Environmental Pollution-
 |
| Environmental Pollution and Climate change |
Air pollution
Air pollution may be defined as “the excessive discharge of undesirable foreign substances into the atmospheric air, thereby adversely affecting the quality of air, and causing damage to human, plants and animal lives.
Types of air pollutant
· Gases: SO2, SO3, H2S, NO, NO2, CO, CO2
· Particulate: Dust, Smoke, Smog
Sulphur dioxide (SO2)
The sources of Sulphur
1. Coal used for thermal power generation
2. Petroleum oil refineries
3. Sulphuric acid plants
Ill effects
1. It causes cardiac and respiratory diseases to man.
2. Cell membrane of plants is damaged.
3. It causes metabolic disorder, Chlorophyll destruction and growth yield reduction.
4. SO2 is source of acid rain which damages vegetation.
Sulphur trioxide (SO3)
The sources of Sulphur
Ill effects
1. It causes breathing uncomfort and irritation to the respiratory tract.
2. SO3 is source of acid rain which damages vegetation.
Hydrogen sulphide (H2S)
H2S is produced from decomposition of sewage waste and organic matter.
Ill effects: It blackens lead paints and causes corrosion of metal.
Nitrogen oxides (NO, NO2)
They are produced from
1. Combustion of fuels such as coal, diesel, petrol, etc.
2. Acid industries, explosive industries.
Ill effects
1. Respiratory illness among children and irritation of eyes
2. Nitrogen oxides and hydrocarbon forms smog. Smog limits the visibility and causes breathing disorder.
3. Nitrogen oxides with moisture forms acid rain.
Carbon monoxide (CO)
Sources of carbon monoxide are:
1. Partial combustion of fuels
2. Automobiles, industries, oil refineries.
3. Domestic heat appliance (where organic fuels are used)
Ill effects
1. It is colourless, odourless but very toxic.
2. CO combines with hemoglobin to form carboxy- hemoglobin; it reduces the oxygen carrying capacity of blood.
3. CO causes laziness, headache, and adverse effect on cardio-vascular system.
Carbon dioxide (CO2)
Sources of carbon monoxide are:
1. Combustion of hydrocarbon fuels
2. Automobiles, industries, oil refineries.
3. Domestic heat appliance (where organic fuels are used)
Ill effects
1. Excessive CO2 causes respiratory disorders and suffocation.
2. Excess CO2 is responsible for global warming.
Particulates
Dust: The main sources of dust are mines and quarries, power houses, cement factories, vehicular traffic, natural wind, rubber tyres abrasion, collides, etc.
Ill effects
1. It causes allergic and respiratory diseases, silicosis (if dust contain silica)
2. Dust causes corrosion and soiling.
Smoke: Smoke is composed of tiny particle of carbon, ash, oil, etc. Incomplete combustion of fuel generates smoke. Combustion of hydrocarbon fuels, Automobiles, industries, oil refineries, Domestic heat appliance.
Ill effect
1. Spoiling of clothing.
2. Damaging exterior finishes of building.
3. Causes respiratory disorders.
Smog: Smog is a blend of smoke and fog in suspended droplet form.
Ill effect
1. Smog reacts with SO2 and SO3, gives sulphuric acid aerosol (London
2. Smog causes poor atmospheric visibility.
Ozone layer depletion
Formation of ozone layer
The ozone layer present in the atmosphere acts as a protective shield for the life on earth. It strongly absorbs UV radiations from the sun in the region 220 to 300 nm and thereby protects the life on earth from severe radiation damage.
Ozone is formed in the stratosphere by the photochemical reaction:
The third body absorbs excess energy and thus stabilizes the ozone molecule. Thus ozone molecule is constantly formed in the atmosphere. However, it is also destroyed by reactions with:
Depletion of ozone
a) Nitric oxide
c) Reactive hydroxyl radical
d) Chlorine released by chlorofluorocarbons.
CFC’s are used as coolants in refrigerators, air conditioners, and propellants, in aerosol sprays and in plastic foams such as thermocol or Styrofoam. The CFC molecule escaping into the environment decomposes to release chlorine in the ozone layer and each atom of chlorine thus liberated is capable of attacking several ozone molecules.
Which generates chlorine atoms so that a long chain process is involved which conserves chlorine atom.
Consequences
1. With the depletion of atmospheric ozone there is a danger of the increase in the flux of UV radiations over earth’s biosphere. All the known effects of these radiations are harmful for human life.
2. These UV radiations slowly cause damage to the eyes, lungs, hairs, skin, etc.
3. These radiations can cause other serious diseases like skin cancer, the blood cancer.
4. “Photochemical smog” is the major cause of ozone exposure.
5. The increased concentration of ozone brings about the changes in DNA and RNA and finally cell death.
Acid rain
Formation of acid rain
The term acid rain means the presence of excessive acids in the rain waters. Acid rain is a mixture of H2SO4 and HNO3. Various industries, automobiles, etc. release acidic oxides such as sulfur dioxide, nitrogen dioxide, hydrogen fluoride, etc. into the atmosphere. These oxides dissolve in moisture present in the atmosphere to form corresponding acids which then fall slowly on earth as acid rain.
Harmful effects
1. Increases acidity of rainwater.
2. Causes damage to freshwater life
3. Causes damage to plants.
4. Changes the rate of metabolism of organisms
5. Causes irritation to eyes and mucous membrane
6. Accelerates the rate of corrosion of metal.
7. Causes damage to buildings, rocks, sculptures, etc.
8. Dissolves salts in soils like CaCO3, metals which passes into water bodies and causes toxicity to aquatic life.
Greenhouse effect
The earth is heated by sunlight and some of the heat that is absorbed by the earth is radiated back into space. However, some of the gases in the lower atmosphere acting like glass allow the incoming solar radiations (in the range of 300 – 2500nm) but do not allow the earth to radiate the heat back into space. In other words, these gases in the atmosphere are transparent to sunlight coming in but they strongly absorb the infrared radiation, which the earth sends back as heat. This result into the heating of the earth surface by this phenomenon called the “Green House Effect”.
Greenhouse gases and Global warming
The gases responsible for greenhouse effect are CO2, water vapor, CH4 and man-made CFC’s. In fact, greenhouse gases, particularly CO2 and water vapor are responsible for keeping our planet warm and thus sustaining life on earth. If the greenhouse gases are very less or totally absent in the atmosphere then the average temperature on earth would have been at sub-zero level. But if the concentration of the greenhouse gases is larger, they may trap too much heat which can lead to excessive heating of the earth, i.e. global warming.
Consequences
1. Rise in temperature on earth surface due to the high level of CO2.
2. Adverse effect on world food production.
3. Rise in sea level due to the melting of polar ice caps.
4. Decrease in biological productivity of oceans.
Minimization
1. Reduction in the use of fossil fuel.
2. Use of alternate sources of energy.
3. Conservation of forests, extensive aforestation.
4. Reduction in use of automobiles and development of more efficient automobile engines.
5. Ban on CFC’s and nuclear explosions.
Photochemical smog
When the atmosphere is loaded with large quantities of automobile exhaust during the warm sunny day with gentle winds at low-level inversion then the exhaust gases are trapped by inversion layers with strongest air masses and simultaneously exposed to intense sunlight.
Then a number of a photochemical reaction involving NO2, HC and eight other organic free radicals takes place leading to the formation of O3, Peroxide and other photochemical oxidants. Photochemical smog’ characterized by the formation of aerosol that reduces visibility, generation of brown hazy fumes that irritate the eyes & lungs & cause extensive damage to vegetation and rubber goods.
PAN – was observed in Los Angeles and Denver in the USA
Ill effect
1. Photochemical smog causes irritation to eyes and lungs
2. It increases the chances of an asthmatic attack.
3. May damage plant.
Remedy
1. Decreasing the nitrogen oxides and hydrocarbon level in the air.
2. Applying catalytic converter to exhaust of automobiles.
Effects of common air pollutants on the human being
The common air pollutants and their effects are:
1. CO: Toxicity, blood poisoning by forming carboxyhemoglobin, which is useless for respiratory purpose and hence leads to death.
2. Oxides of Nitrogen: Respiratory irritation, headache, bronchitis, impairment of lung, loss of appetite, corrosion of teeth.
3. Oxides of sulfur: Suffocation, aggravation of asthma and chronic bronchitis, impairment of pulmonary functions, respiratory functions and sensory irritation of throat and eyes.
4. Hydrocarbons and particulate matter: Carcinogenic, causes breathing problems, irritation of lungs, pulmonary fibrosis, asbestosis, etc.
Pollution Control
1. Emission of automobiles and vehicles may be minimized by cleaning exhaust gases after combustion by using a catalytic converter.
Catalytic converter
A catalytic converter is a device used to reduce the toxicity of emissions from an internal combustion engine. Catalytic converters are also used on generator sets, forklifts, mining equipment, trucks, buses, trains, and other engine-equipped machines. A catalytic converter provides an environment for a chemical reaction wherein toxic combustion by-products are converted to less-toxic substances.
A three-way catalytic converter has three simultaneous tasks:
i. Reduction of nitrogen oxides to nitrogen and oxygen: 2NOx → xO2 + N2
ii. Oxidation of carbon monoxide to carbon dioxide: 2CO + O2 → 2CO2
iii. Oxidation of unburnt hydrocarbons (HC) to carbon dioxide and water:
CxH2x+2 + 2xO2 → xCO2 + 2xH2O
Catalytic converters are used on spark ignition (gasoline; liquefied petroleum gas, LPG); flexible fuel vehicles burning varying blends of E85 and gasoline; compressed natural gas (CNG)) engines; and compression ignition (diesel) engines.
For spark ignition engines, the most commonly used catalytic converter is the three-way converter which converts the three main pollutants of concern — CO, HC, and NOx— to less-toxic substances. The control of NOx involves a reduction process that releases oxygen and the control of CO and HC involves an oxidation process that consumes oxygen. Therefore, a 3-way converter contains two catalyst-coated stages: The first catalyst stage encountered by the exhaust is for reduction of NOx, which produces oxygen.
2. The use of tall stacks or chimneys reduces the concentration of air pollutants at the ground level.
3. The cyclone collector: Cyclone collector work on the principle centrifugal force. Due to this force heavy particulates flow towards the wall of the chamber and settled down due to gravity. Cyclone collector removes smoke and dust of 5-20 µm size.
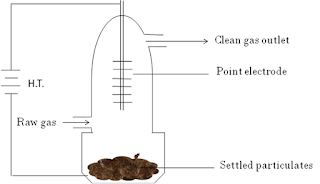
4. Cottrell electrostatic precipitator
Smoke is the colloidal of negatively charged carbon particles in air. Before passing the smoke to the chimney, it is sent through a chamber provided with a knob maintained at very high potential of 30,000 V. Under the influence of strong electric field, smoke particles get robbed of their negative charge. Due to this smoke particles are precipitated out and settled at the bottom of chamber. The hot gases are escaped through chimney.
5. Elimination of dust: For this purpose the extraction ventilation is applied. The air steam carrying suspended dust is first maintained at a sufficient velocity to keen dust particle in suspension. Thereafter, the rate is reduced suddenly to the extent to cause the dust particles to settle down in a stilling chamber.







Comments
Post a Comment